Interpretation of NMR Results
Contents
Introduction
Nuclear Magnetic Resonance, NMR, is like every other spectroscopic technique - it uses electromagnetic radiation to probe the structure of molecules. In UV and IR it is bonds that are studied, in NMR it is nuclei of certain elements. The main element that is studied in NMR is hydrogen, fortunately hydrogen is in almost every organic compound, which makes NMR extremely useful in fields from pharmaceuticals and agrichemicals to the petroleum industry. In the NMR literature hydrogen is called proton, hence the name proton NMR. A proton NMR spectrum will only show protons, no other elements. Similarly, a carbon spectrum will show only carbons. The other major nuclei that can be studied with NMR are nitrogen, phosphorus and fluorine. Each nucleus requires special tuning of the instrument to be able to see.
What does the result look like?
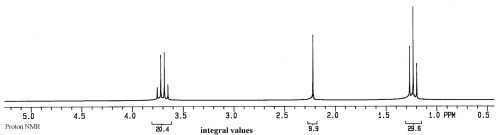
If a sample has just a single compound with a concentration of 1-10mg/ml, a proton NMR spectrum will look similar to:
This spectrum is of ethanol. The Y axis is relative intensity, while the X axis is relative frequency. Instead of using Hz for frequency, for historical reasons the unit is called ppm, or parts per million, and the scale begins at 0 from the right side and increases in ppm to the left. The normal range for organic compounds is from about 0.5 to 15 ppm in proton NMR spectra.
Peaks
Based on the current theories, each peak in an NMR spectrum represents a different, magnetically distinct proton in the sample. Of course some peaks could overlap, but they will still be magnetically unique.