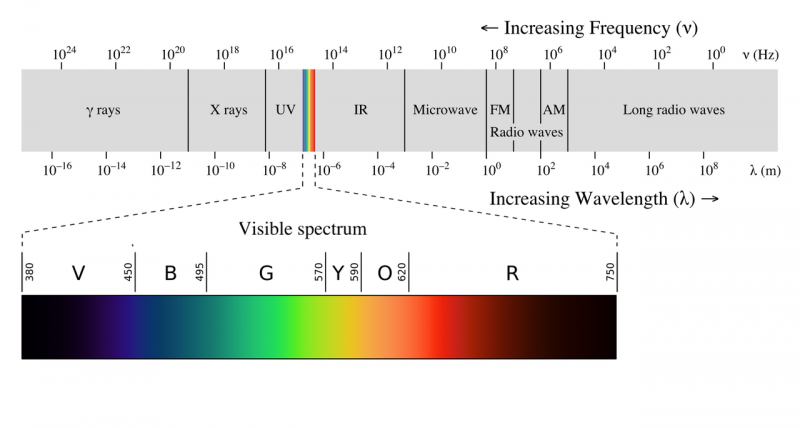
Electromagnetic spectrum
Jump to navigation
Jump to search
The electromagnetic spectrum is often shown as a range of photon energies and their names. Since, for electromagnetism, energy = frequency times a constant, as frequency goes up the energy of a photon goes up, so the highest energy photons have the highest frequency. Apparently the equation E=hv was just a guess by Max Plank, at the time there was no experimental or theoretical justification for the equation. A photon with a frequency of 1 Hz would thus have an energy of h times 1/second which is equal to 6.62607015×10−34 Joules, while a single photon of a 1024 Hz gamma ray would have 6.6x10-10 joules of energy.
1 joule can be visualized by imagining the amount of energy needed to raise an apple one meter into the air.