Difference between revisions of "Electromagnetic spectrum"
Jump to navigation
Jump to search
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
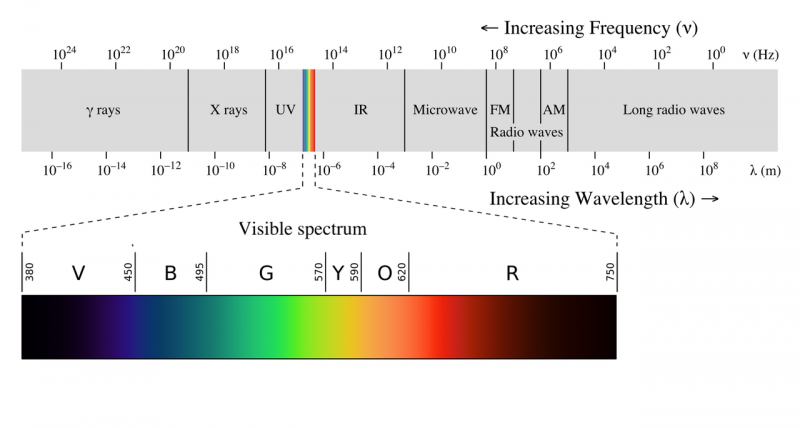
| − | The electromagnetic spectrum is often shown as a range of [[Photons|photon]] energies and their names. Since, for electromagnetism, energy = frequency times a constant, as frequency goes up the energy of a photon goes up, so the highest energy photons have the highest frequency | + | The electromagnetic spectrum is often shown as a range of [[Photons|photon]] energies and their names. Since, for electromagnetism, energy = frequency times a constant, as frequency goes up the energy of a photon goes up, so the highest energy photons have the highest frequency. Apparently the equation E=hv was just a guess by Max Plank, at the time there was no experimental or theoretical justification for the equation. A photon with a frequency of 1 Hz would thus have an energy of h times 1/second which is equal to 6.62607015×10<sup>−34</sup> Joules, while a single photon of a 10<sup>24</sup> Hz gamma ray would have 6.6x10<sup>-10</sup> joules of energy. |
[[File:EM_spectrum.png|800px]] | [[File:EM_spectrum.png|800px]] | ||
| + | |||
| + | |||
| + | 1 joule can be visualized by imagining the amount of energy needed to raise an apple one meter into the air. | ||
Latest revision as of 14:57, 22 March 2020
The electromagnetic spectrum is often shown as a range of photon energies and their names. Since, for electromagnetism, energy = frequency times a constant, as frequency goes up the energy of a photon goes up, so the highest energy photons have the highest frequency. Apparently the equation E=hv was just a guess by Max Plank, at the time there was no experimental or theoretical justification for the equation. A photon with a frequency of 1 Hz would thus have an energy of h times 1/second which is equal to 6.62607015×10−34 Joules, while a single photon of a 1024 Hz gamma ray would have 6.6x10-10 joules of energy.
1 joule can be visualized by imagining the amount of energy needed to raise an apple one meter into the air.