Difference between revisions of "Electromagnetic spectrum"
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
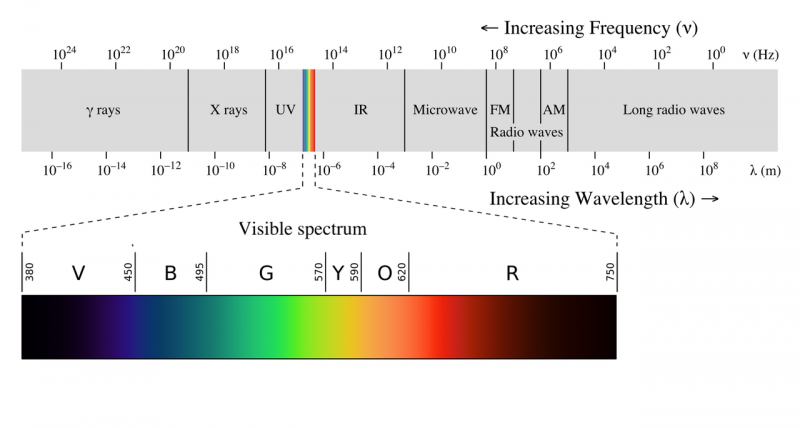
| − | The electromagnetic spectrum is often shown as a range of [[Photons|photon]] energies and their names. Since energy = frequency*speed of light and speed of light is a constant, as frequency goes up the energy of a photon goes up. So the highest energy photons have the highest frequency but the shortest wavelength. | + | The electromagnetic spectrum is often shown as a range of [[Photons|photon]] energies and their names. Since energy = frequency*speed of light and speed of light is a constant, as frequency goes up the energy of a photon goes up. So the highest energy photons have the highest frequency but the shortest wavelength. Apparently the equation E=hv was just a guess by Max Plank, at the time there was no experimental or theoretical justification for the equation. |
[[File:EM_spectrum.png|800px]] | [[File:EM_spectrum.png|800px]] | ||
Revision as of 14:17, 22 March 2020
The electromagnetic spectrum is often shown as a range of photon energies and their names. Since energy = frequency*speed of light and speed of light is a constant, as frequency goes up the energy of a photon goes up. So the highest energy photons have the highest frequency but the shortest wavelength. Apparently the equation E=hv was just a guess by Max Plank, at the time there was no experimental or theoretical justification for the equation.