Difference between revisions of "Electromagnetic spectrum"
Jump to navigation
Jump to search
| Line 1: | Line 1: | ||
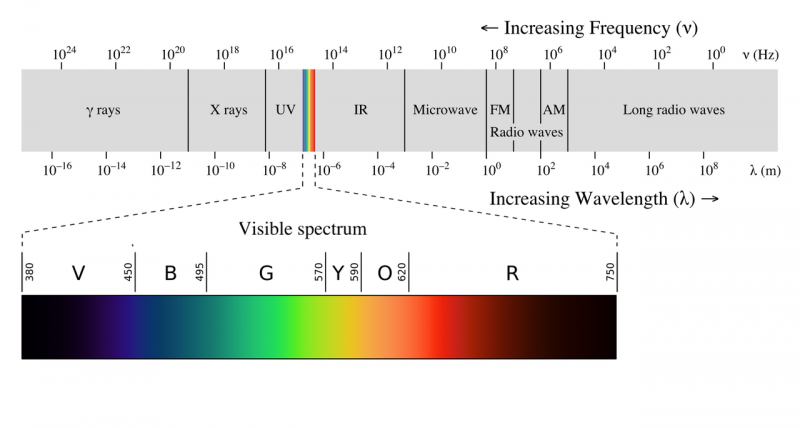
The electromagnetic spectrum is often shown as a range of photon energies and their names. Since energy = frequency*speed of light and speed of light is a constant, as frequency goes up the energy of a photon goes up. So the highest energy photons have the highest frequency but the shortest wavelength. | The electromagnetic spectrum is often shown as a range of photon energies and their names. Since energy = frequency*speed of light and speed of light is a constant, as frequency goes up the energy of a photon goes up. So the highest energy photons have the highest frequency but the shortest wavelength. | ||
| − | [[File:EM_spectrum.png| | + | [[File:EM_spectrum.png|800px]] |
Revision as of 19:46, 18 March 2020
The electromagnetic spectrum is often shown as a range of photon energies and their names. Since energy = frequency*speed of light and speed of light is a constant, as frequency goes up the energy of a photon goes up. So the highest energy photons have the highest frequency but the shortest wavelength.