Difference between revisions of "Interpretation of NMR Results"
(→Peaks) |
|||
| Line 11: | Line 11: | ||
==Peaks== | ==Peaks== | ||
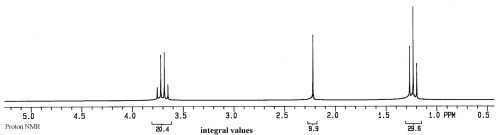
| − | Based on the current [[NMR Theories|theories]], each peak in an NMR spectrum represents a different, magnetically distinct proton in the sample. Of course some peaks could overlap, but they will still be magnetically unique. Because of coupling between the protons in a molecule there is often splitting of their peak into what are called multiplets. In the ethanol spectrum above, the quartet at 3.7ppm is due to the CH<sub>2</sub>, the triplet at 1.25ppm is due to the CH<sub>3</sub>, and the singlet at 2.2ppm is due to the hydroxyl proton. In each case, if the sample is pure, a peak will correspond to a magnetically unique proton or group of protons. Thus the value of NMR is that it can provide extremely detailed information about a sample. | + | Based on the current [[NMR Theories|theories]], each peak in an NMR spectrum represents a different, magnetically distinct proton or group of protons in the sample. Of course some peaks could overlap, but they will still be magnetically unique. Because of coupling between the protons in a molecule there is often splitting of their peak into what are called multiplets. In the ethanol spectrum above, the quartet at 3.7ppm is due to the CH<sub>2</sub>, the triplet at 1.25ppm is due to the CH<sub>3</sub>, and the singlet at 2.2ppm is due to the hydroxyl proton. In each case, if the sample is pure, a peak will correspond to a magnetically unique proton or group of protons. Thus the value of NMR is that it can provide extremely detailed information about a sample. |
For instance, there are only 3 peaks, so we know for a fact that there are only 3 groups of protons in the molecules of the sample. We also know that, based on the positions of the peaks, there are no aldehydes or aromatic protons in the sample. We also know that the magnetic environments around the three groups of protons are clean and that the molecules are probably not aggregated. Aggregation gives more structure to a solution, which would change local magnetic environments and cause more peaks to appear, such as having two quartets near 3.5ppm. | For instance, there are only 3 peaks, so we know for a fact that there are only 3 groups of protons in the molecules of the sample. We also know that, based on the positions of the peaks, there are no aldehydes or aromatic protons in the sample. We also know that the magnetic environments around the three groups of protons are clean and that the molecules are probably not aggregated. Aggregation gives more structure to a solution, which would change local magnetic environments and cause more peaks to appear, such as having two quartets near 3.5ppm. | ||
Revision as of 08:23, 1 May 2020
Contents
Introduction
Nuclear Magnetic Resonance, NMR, is like every other spectroscopic technique - it uses electromagnetic radiation to probe the structure of molecules. In UV and IR it is bonds that are studied, in NMR it is nuclei of certain elements. The main element that is studied in NMR is hydrogen, fortunately hydrogen is in almost every organic compound, which makes NMR extremely useful in fields from pharmaceuticals and agrochemicals to the petroleum industry. In the NMR literature hydrogen is called proton, hence the name proton NMR. A proton NMR spectrum will only show protons, no other elements. Similarly, a carbon spectrum will show only carbons. The other major nuclei that can be studied with NMR are nitrogen, phosphorus and fluorine. Each nucleus requires special tuning of the instrument to be able to see.
What does the result look like?
If a sample has just a single compound with a concentration of 1-10mg/ml, a proton NMR spectrum will look similar to:
This spectrum is of ethanol. The Y axis is relative intensity, while the X axis is relative frequency. Instead of using Hz for frequency, for historical reasons the unit is called ppm, or parts per million, and the scale begins at 0 from the right side and increases in ppm to the left. The normal range for organic compounds is from about 0.5 to 15ppm in proton NMR spectra.
Peaks
Based on the current theories, each peak in an NMR spectrum represents a different, magnetically distinct proton or group of protons in the sample. Of course some peaks could overlap, but they will still be magnetically unique. Because of coupling between the protons in a molecule there is often splitting of their peak into what are called multiplets. In the ethanol spectrum above, the quartet at 3.7ppm is due to the CH2, the triplet at 1.25ppm is due to the CH3, and the singlet at 2.2ppm is due to the hydroxyl proton. In each case, if the sample is pure, a peak will correspond to a magnetically unique proton or group of protons. Thus the value of NMR is that it can provide extremely detailed information about a sample.
For instance, there are only 3 peaks, so we know for a fact that there are only 3 groups of protons in the molecules of the sample. We also know that, based on the positions of the peaks, there are no aldehydes or aromatic protons in the sample. We also know that the magnetic environments around the three groups of protons are clean and that the molecules are probably not aggregated. Aggregation gives more structure to a solution, which would change local magnetic environments and cause more peaks to appear, such as having two quartets near 3.5ppm.